
Syringe Assembly Machines - labeling, plunger rod, finger flange and safety device
When it comes to processing pharmaceutical packaging containers, it is essential to handle them with utmost care to maintain their integrity and quality. To ensure this, groninger syringe assembly machines as well as for vials and cartridges come equipped with a non-contact transfer system that gently processes the primary packaging without compromising its quality.
The modular and compact design of the system makes it highly adaptable to your specific requirements and packaging needs. Additionally, it can easily be expanded with additional process modules, offering you even more flexibility and customization options.
With groninger assembly solutions, you can be confident that your primary packaging will be processed with the utmost care and precision, ensuring the highest product quality standards for your pharmaceutical products.
Process steps of the syringe assembly machines
Plunger Rod
In the plunger rod insertion process, it is critical to ensure the integrity of the pharmaceutical packaging and plunger stopper. groninger has a patented on the market to process and insert the plunger rod, which prevents the plunger stopper from being displaced axially or rotated in the syringe when the plunger rod is inserted. All the parameters required for this can be set and saved in the recipe management. As a result, groninger offers a safe and reliable solution for your insertion process.

Labeling
Labels are an important carrier of information and, therefore, are very important for pharmaceutical products. Consequently, it is all the more important that the label is printed with the correct information and affixed in the intended location. groninger guarantees extremely precise and gentle labeling of different pharmaceutical primary packaging materials. Labels that have been detected as faulty are not applied to the packaging. All primary packaging is provided with an error-free label and labeled.

Backstop
The backstop prevents that the plunger stopper is unintentionally pulled out from the syringe barrel. In addition to this brake function, the backstop prevents the twisting of the plunger rod, so that the plunger rod cannot be unscrewed unintentionally during application. On top of that, with the help of the backstop, it creates an improved finger positioning. With the groninger solutions, the backstops are fully and carefully assembled on the flange of the syringe to ensure the basic functions of the backstop and preventing any damage to the glass or plastic syringes.
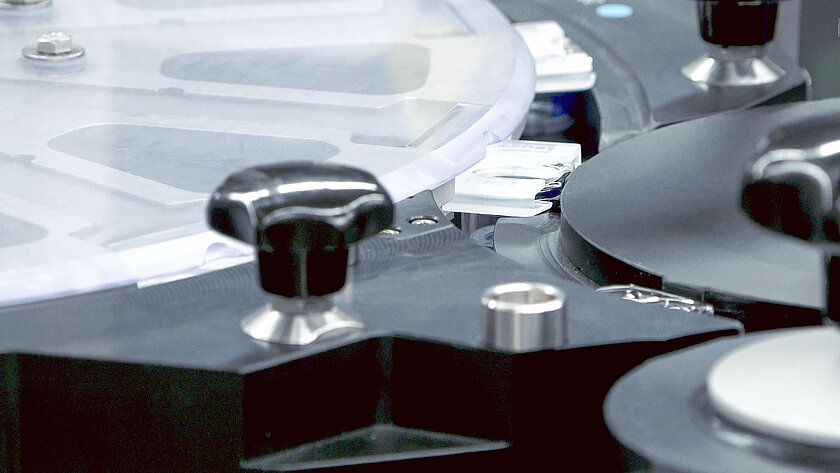
Safety Device
Billions of injections are administred around the world every year. Unfortunately, with such a large number of injections, there are numerous needlestick injuries that may result in infectious diseases. Therefore, it is all the more important to assemble prefillable syringes with safety components or safety devices. From semi-automated lab equipment to high-speed for safety devices, groninger has everything in its downstream processing portfolio.
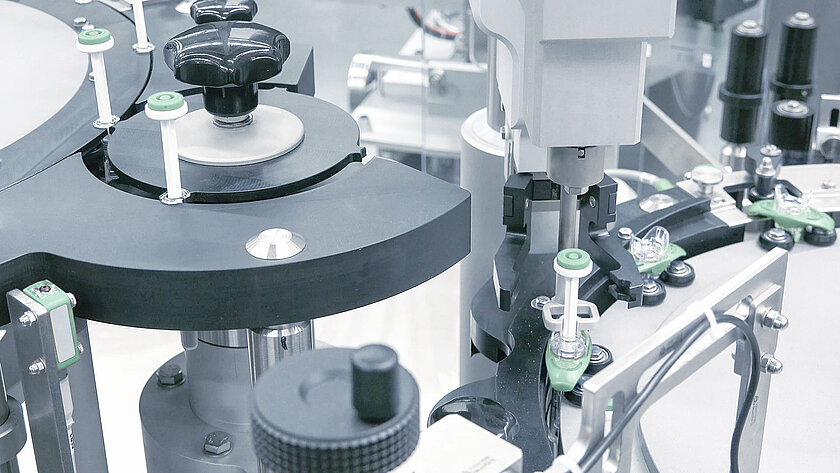
Downstream equipment for pre-filled syringes, vials and cartridges
In downstream processing, careful and high-quality processing of the primary packaging is of immense importance. Therefore, groninger assembly machines can be equipped with a non-contact transport for the primary packaging.
Due to the modular and compact design, the lines can be flexibly adapted to your requirements and packaging and can be attached to existing groninger lines. The expansion of additional process functions is optionally possible at any time.
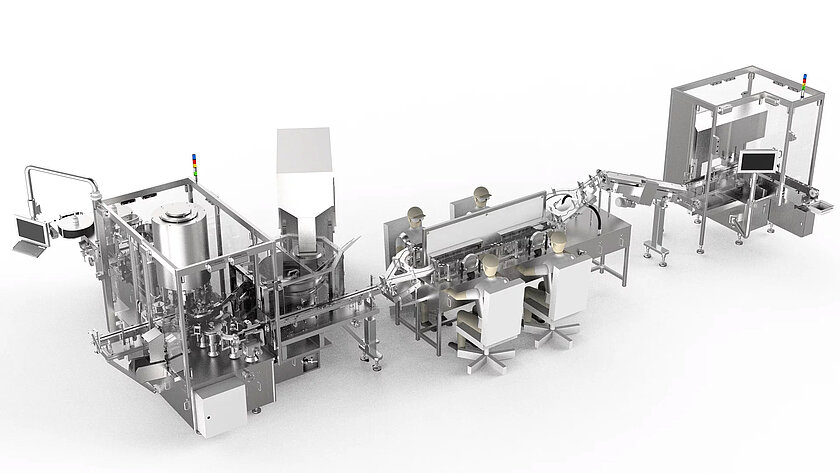
Up to 70 objects per minute
- 3 in 1 processing of all common syringes, vials and cartridges
- Modular expansion of backstop, finger flange and safety device assembly
- Patented electromotive plunger rod assembly
- Integrated removal of bad labels without object loss
- Different inspection methods, sensors/image processing systems
- Integrating all common printers
- Handling systems for feeding, tray loading and buffering
Up to 300 objects per minute
- 3 in 1 processing of all common packaging containers
- Modular expansion of backstop, finger flange and safety device assembly
- Patented electromotive plunger rod assembly
- Integrated removal of bad labels without loss of object
- Different inspection methods, sensors/image processing systems
- Integration of all common printers
- Automatic splicing unit at full capacity
- Handling lines for feeding, tray loading and buffering
Up to 600 objects per minute
- 3 in 1 processing of all common packaging containers
- Modular expansion of backstop, finger flange and safety device assembly
- Patented electromotive plunger rod assembly
- Integrated removal of bad labels without loss of object
- Different inspection methods, sensors/image processing systems
- Integration of all common printers
- Automatic splicing unit at full capacity
- Handling lines for feeding, tray loading and buffering
