
Washing and assembling RTU primary packaging containers for the pharmaceutical industry
With our washing and assembly lines we cover a large part of these process steps that are necessary for the production of pre-sterilized packaging.
Whether it's washing and depyrogenating the primary packaging containers, using various siliconization options or assembling closure systems and sealing caps, groninger always has the correct line solution.
Process steps during the manufacturing of RTU packaging containers
There are different process steps involved in the production of ready-to-use syringes, vials or cartridges, from feeding the containers to washing and siliconizing to storing them in nests and tubs.
Feeding glass containers
When the prefillable glass containers are fed into the lines they are usually packed on trays. However, the exact packaging, varies depending on the primary packaging and requires a suitable system for feeding or handling.
Our systems takes care of your needs. groninger has several established solutions in its portfolio, from a detray module and a depalletizing module to state-of-the-art robot applications.

Washing
The glass containers are cleaned and dried in a washing module. On our lines, we reduced the media consumption to a minimum and are still able to achieve an excellent washing result up to a 3-log reduced cleaning.
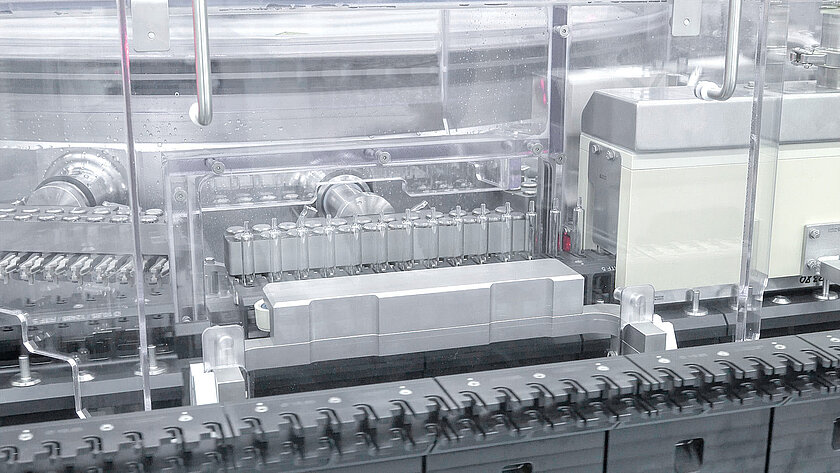
Sterilizing/depyrogenation
The sterilization process developed by groninger attaches great importance to a careful processing method, to easy accessibility of all components for problem-free maintenance, while providing the highest quality and process reliability.
Careful sterilization is already achieved in our innovative sterile tunnel through gentle heating in the inlet area. Quality is also ensured through the use of high-end sterile filters in the sterilization area.
A great advantage of this procedure is the low temperature difference on the object, this characterizes this process. On top of that, it prevents overkill in the sterile process.
The modular structure of the process allows for easy adjustments. At the same time, solutions that do not have a "ring of concern" at the outlet to the sterile area and critical inflatable seals were omitted.

Siliconization
Against the backdrop of increasing quality requirements and the increase in biotechnologically produced, protein-based drugs, an even coating of the surfaces of the glass containers with reduced amounts of silicone oil is necessary.
We have succeeded in minimizing the sprayed silicone oil amounts without affecting the sliding forces in our lines.
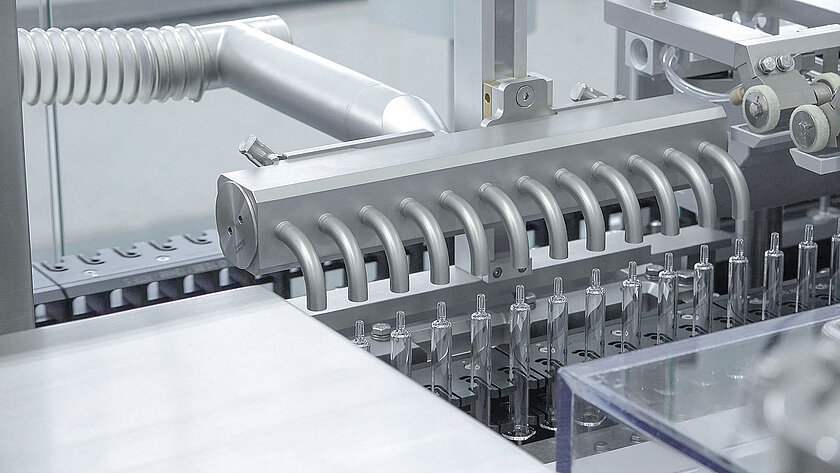
Checking
Control made easy: All lines or process steps can be equipped with different test systems. Sensors, state-of-the-art camera systems and complex X-ray systems can be integrated into the process.
These systems can be used to check geometric parameters and inspect glass containers for cosmetic defects. For example, groninger provides a camera system that checks the finger grip of a syringe for broken glass and the correct contour.
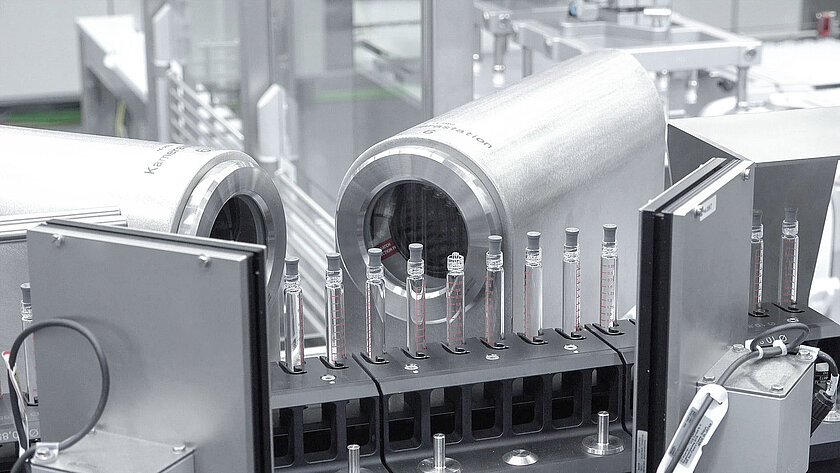
Assembly
One of the most important process steps of these lines is the assembly of various closures, needle guards and caps.
The closures have different properties and tasks. For syringes, the cannula must be protected from damage and kept sterile until the injection. There are also closures that are equipped with a needle protection mechanism, which is intended to protect against injuries on the one hand and prevent accidental reuse on the other.
In the system, all of these components are transported to the respective assembly stations via a specially developed storage and delivery system, where they are assembled on the glass container. Precise assembly of the closures is of the utmost importance.
All assembly processes are subsequently checked by a wide variety of inspection systems.

Tray loading
The processed and closed glass containers can then be used in a wide variety of pharmaceutical end packaging.
A gentle implementation is of great importance, here too the glass containers must not touch each other throughout the entire process. An inspection system can then verify that the final packaging has been completely filled.

Excellent line concepts for washing and assembling pharmaceutical RTU primary packaging containers
Regardless of which pharmaceutical primary packaging you would like to produce for your portfolio, we can provide you with a suitable processing and line concept for washing, siliconizing and assembling ready-to-use packaging containers. groninger is also able to respond to your individual requirements when processing pharmaceutical packaging. An example is the assembly module, which is able to assemble needle or Luer lock syringes with a wide variety of closure components. With the help of a very small number of format parts, the assembly module can be set up for the desired locking systems or locking components. Thanks to many years of experience in the processing of pharmaceutical primary packaging and an understanding of the packaging and its associated components, groninger is able to provide you with maximum precision in assembly and flexibility within the line concept.
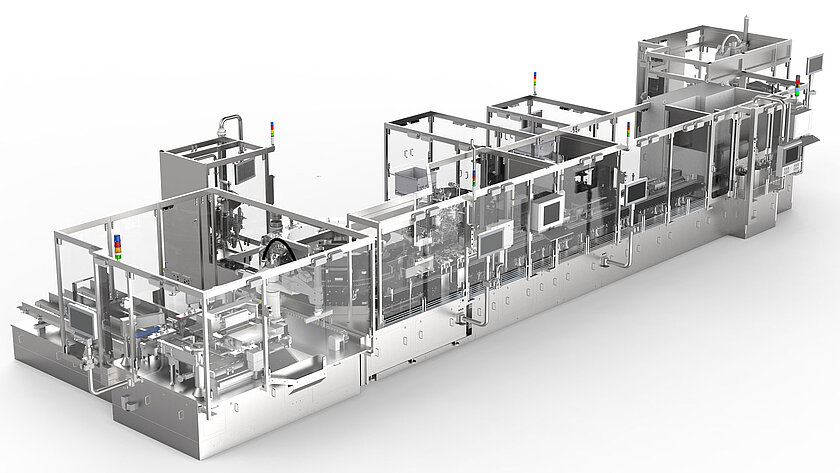
Technical features and highlights of the production lines for RTU syringes, vials and cartridges
- Washing: WFI (water for injection) is used to wash the glass containers. With decades of know-how gained on washing glass containers, groninger ensures that even the highest requirements in the pharmaceutical industry are met.
- Siliconizing: groninger uses its many years of development experience in the field of siliconization to create a homogeneous layer of silicon on the desired surfaces of the glass containers. Patented siliconization methods are used to manufacture sterile syringes and cartridges.
- Careful processing: To avoid glass breakage (and the resulting particles and contamination), the glass containers are processed and transported without contact. It is especially important that the glass containers cannot touch each other throughout the entire line.
- Laminar flow friendliness: The various line concepts and machine modules are designed in such a way that there is no movement above the glass containers. This prevents the possible turbulence of any particles that may be generated and ensures that the glass containers are always under First Air.
- Flexibility: Our lines provide you with maximum flexibility for processing a wide variety of objects and object sizes.
- Highest process reliability: Our systems for washing and assembling RTU syringes, vials and cartridges offer maximum process reliability through a wide variety of camera and inspection options.



