![[Translate to English:] Innovative Lösungen für pharmazeutische Abfüllanlagen](/fileadmin/content/01_Pharma/01_Unsere_Kompetenz/05_Innovative_Loesungen/groninger-smarttrack.jpeg)
Filling and closing syringes, vials and cartridges is even easier, safer and more efficient
Innovative solutions for a changed pharmaceutical industry: The development is moving towards personalized medicines, the approach of blockbusters as "one-size-fits-all" is becoming rarer. At the same time, however, the vast majority of the installed filling systems have been specified for little flexibility but high output.
We have adapted and optimized our machine portfolio to these changed market conditions. In doing so, we had a particular focus on the pharmaceutical processes that can be used on the systems.

smartfill
Minimize product loss
In all areas of biotechnology, it is extremely important that the process-related product loss in aseptic filling lines is minimized. This applies to product development and clinical studies as well as to the production of personalized medicines, for example, for cell and gene therapies.
Under the name smartfill groninger offers process solutions that eliminate product losses during the start-up process, during production and at the end of a batch.
This includes various measures:
- A special priming mode that, in combination with a 100% In-Process-Control (IPC) available as standard, eliminates product losses during start-up.
- The permanent In-Process-Control (IPC) in combination with other measures prevents losses in the production process.
- The special run empty mode ensures that the mechanical filling process does not generate any losses at the end of a batch.
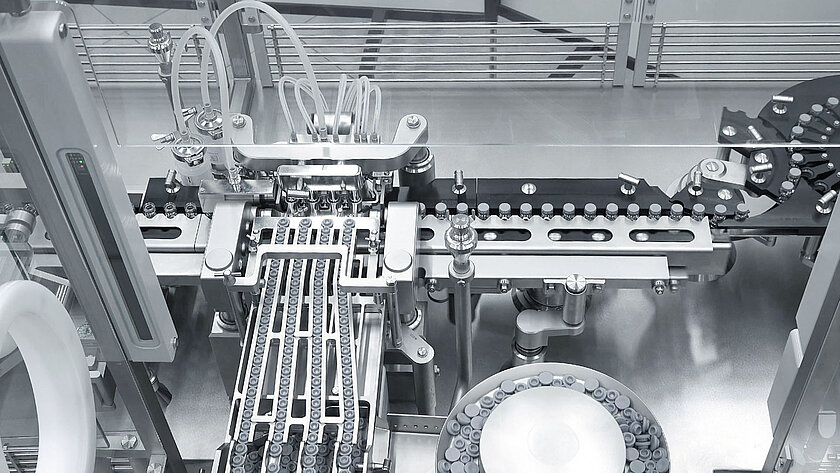
safetransfer
Safe transfer into the aseptic area
With groninger's safetransfer philosophy, it provides a safe process for introducting pre-sterilized components into the aseptic area.
The sterile introduction of RTU packaging into the aseptic processing area is an extremely critical process. From the proven process solutions that can be used depending on the situation and product requirements, groninger offers the right solution for every customer.
Feeding ready-to-use components
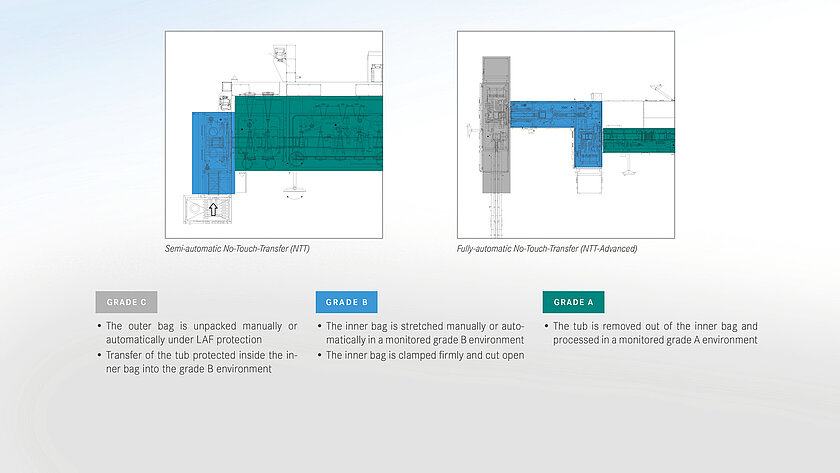
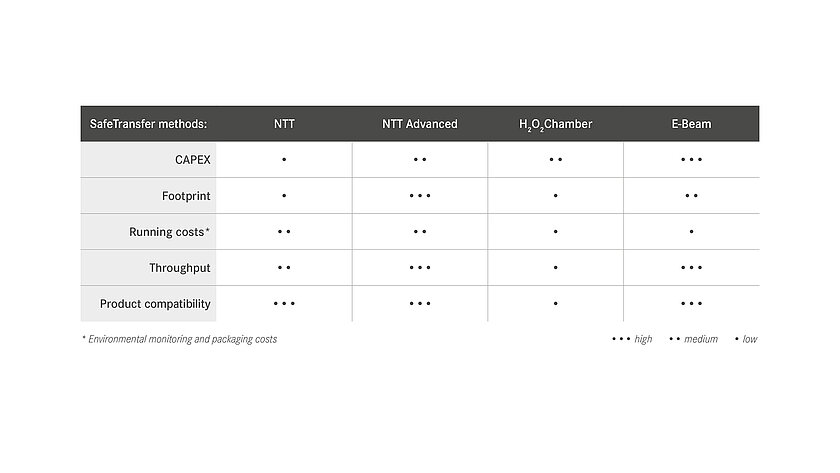
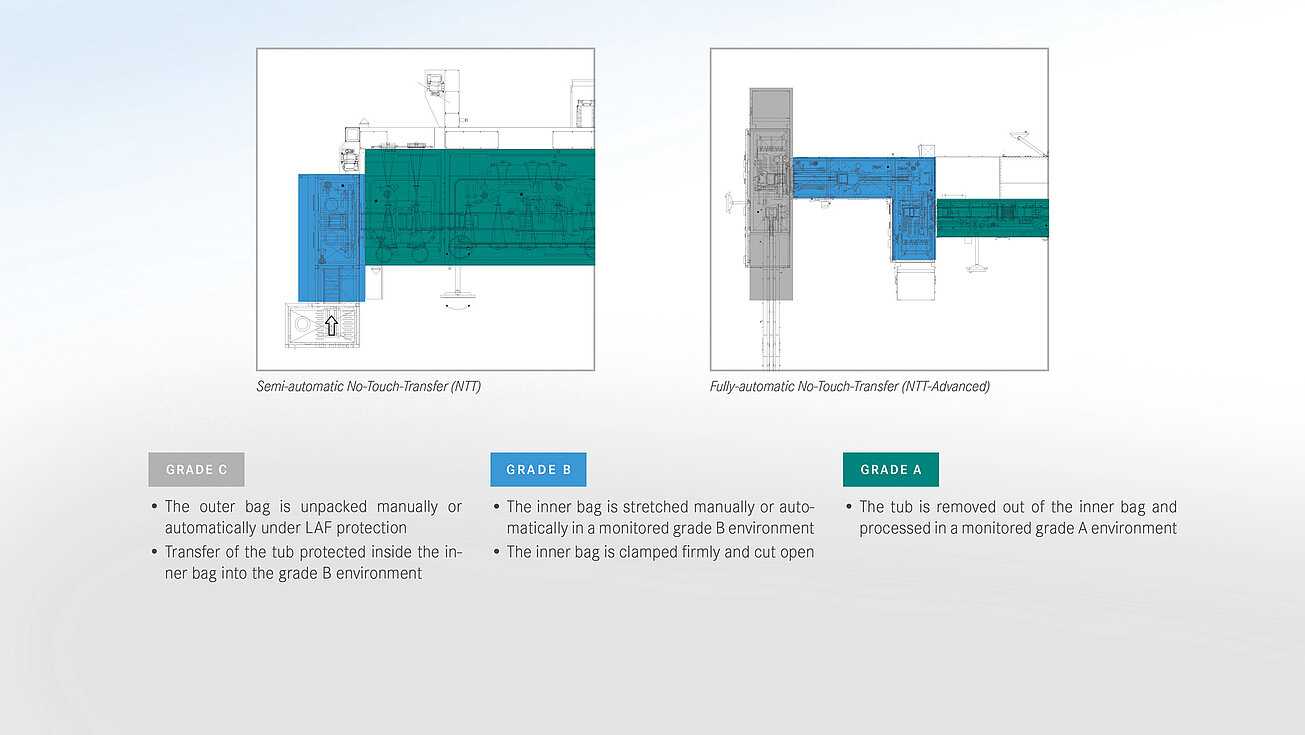
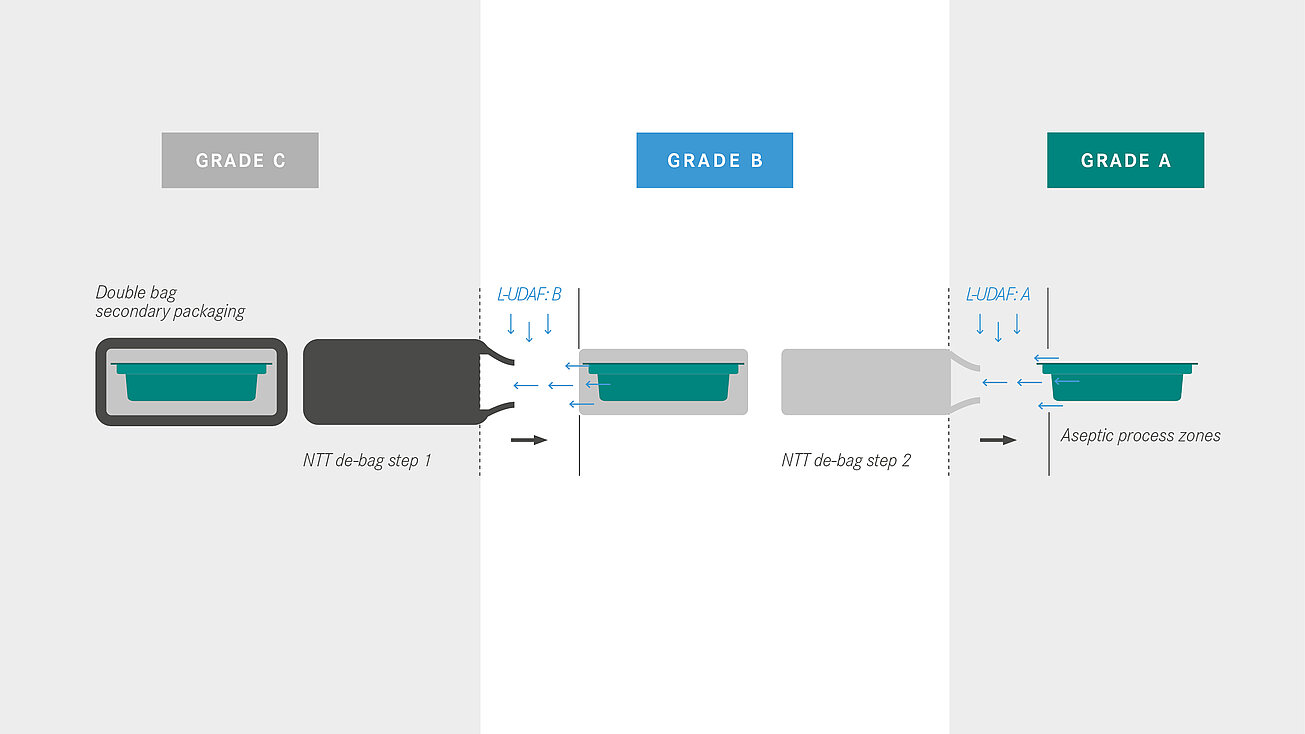
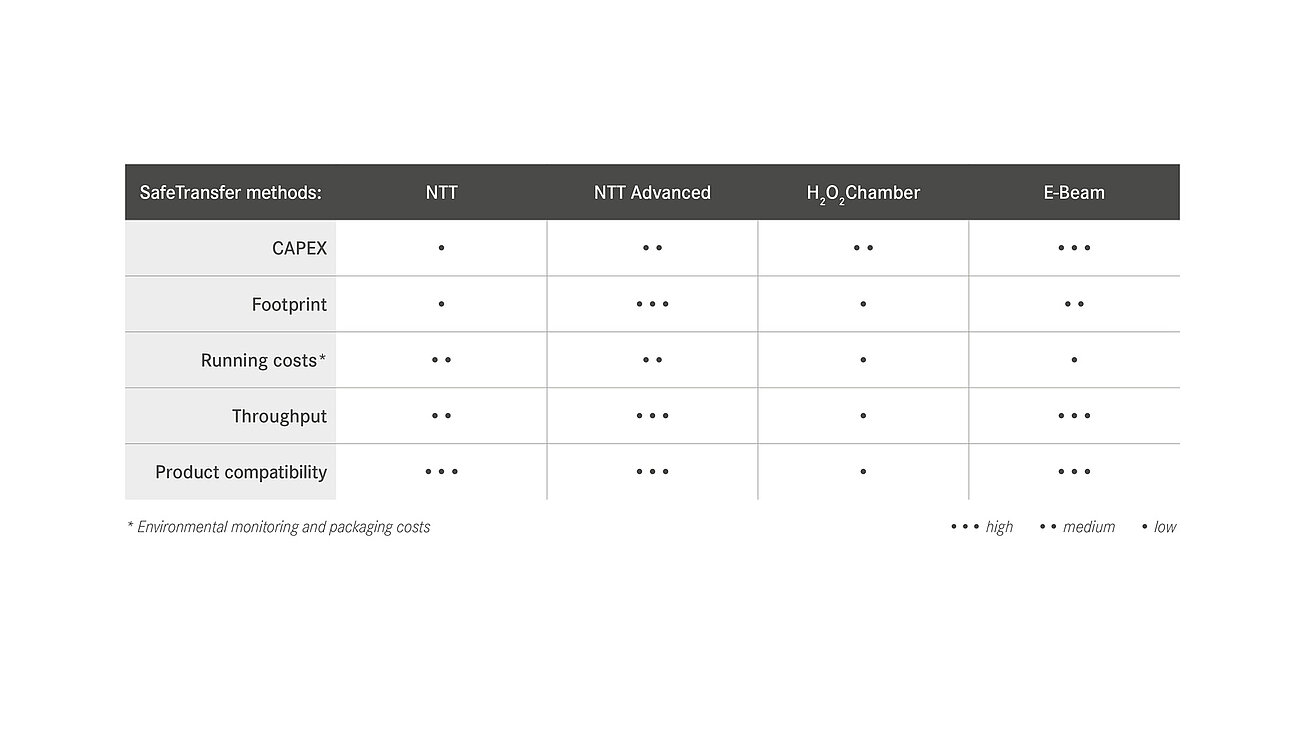
For the aseptic transfer into the A room (isolator, o-RABS or c-RABS), groninger distinguishes between different methods as part of the safetransfer philosophy. The decision for a certain procedure depends on various possible factors:
- batch sizes to be processed,
- products to be processed,
- planned capital expenditures (CAPEX),
- required installation space and
- ongoing costs.
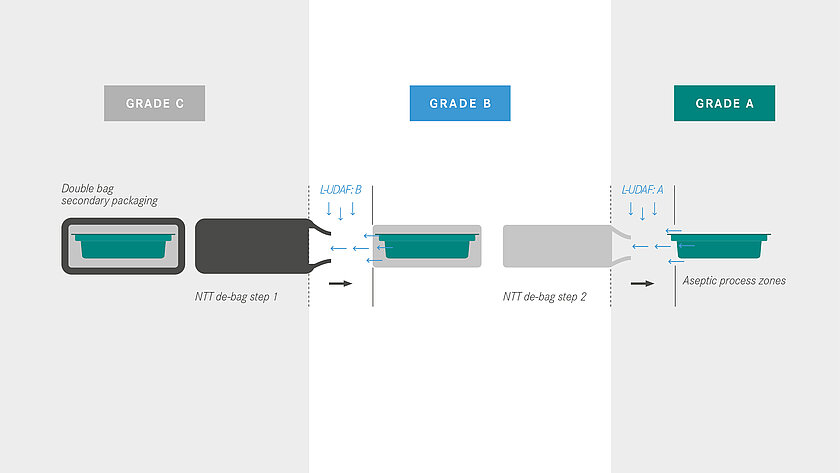
A basic distinction is made between transfer methods with an external decontamination step on the one hand and the processing of double bags, the so-called no-touch transfer (NTT), on the other hand. At the NTT, the primary packaging is delivered sterile and transferred to the aseptic area (Grade A) via a defined process, supported by continuous germ and particle monitoring.
combifill
Easy changeover between formats and packaging
Making diversity possible: With the groninger combifill process solution, you have the option of filling nested ready-to-use syringes as well as vials and cartridges with a minimum number of format parts per system. The decisive factor is that the entire system can be completely changed over to different formats or packaging within a very short time.
With the possibility of operating different filling system on the lines increases your flexibility.
quickconnect
Component change without using tools
Changing components without the use of tools not only reduces set-up time, but also makes aseptic work much easier. With groninger's quickconnect concept, screw connections can be eliminated for all parts that come into direct or indirect contact with the formats or products.
All components or assemblies that have to be removed from a system, for example, to clean them or because the packaging is changing, can be removed with this system without using any tools.
The use of quickconnect can be made easier with an electronically guided installation aid. This not only enables GMP-compliant installation, but also prevents incorrect insertion. In this way, quickconnect saves the user up to 60% of the set-up time compared to traditional lines.

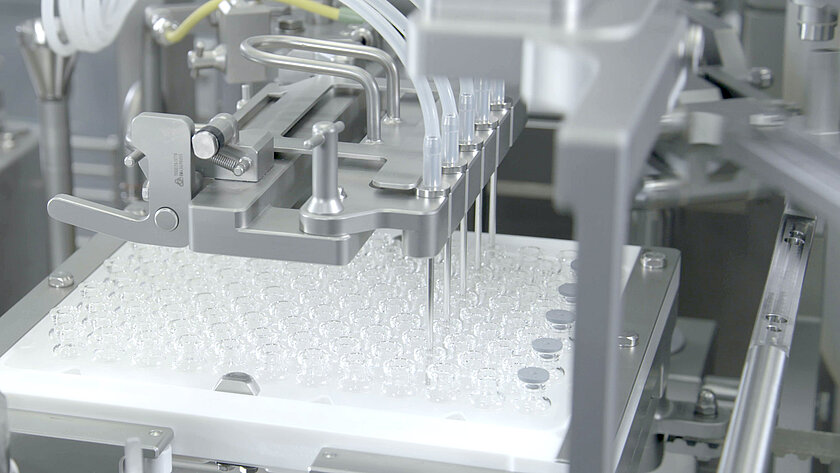
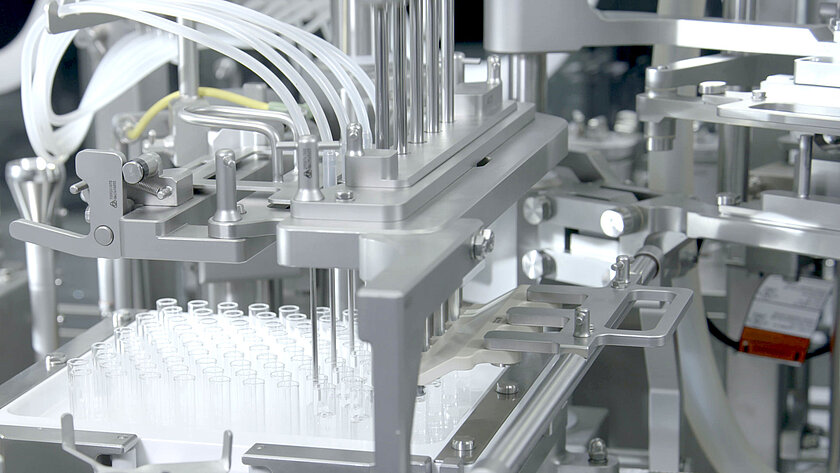
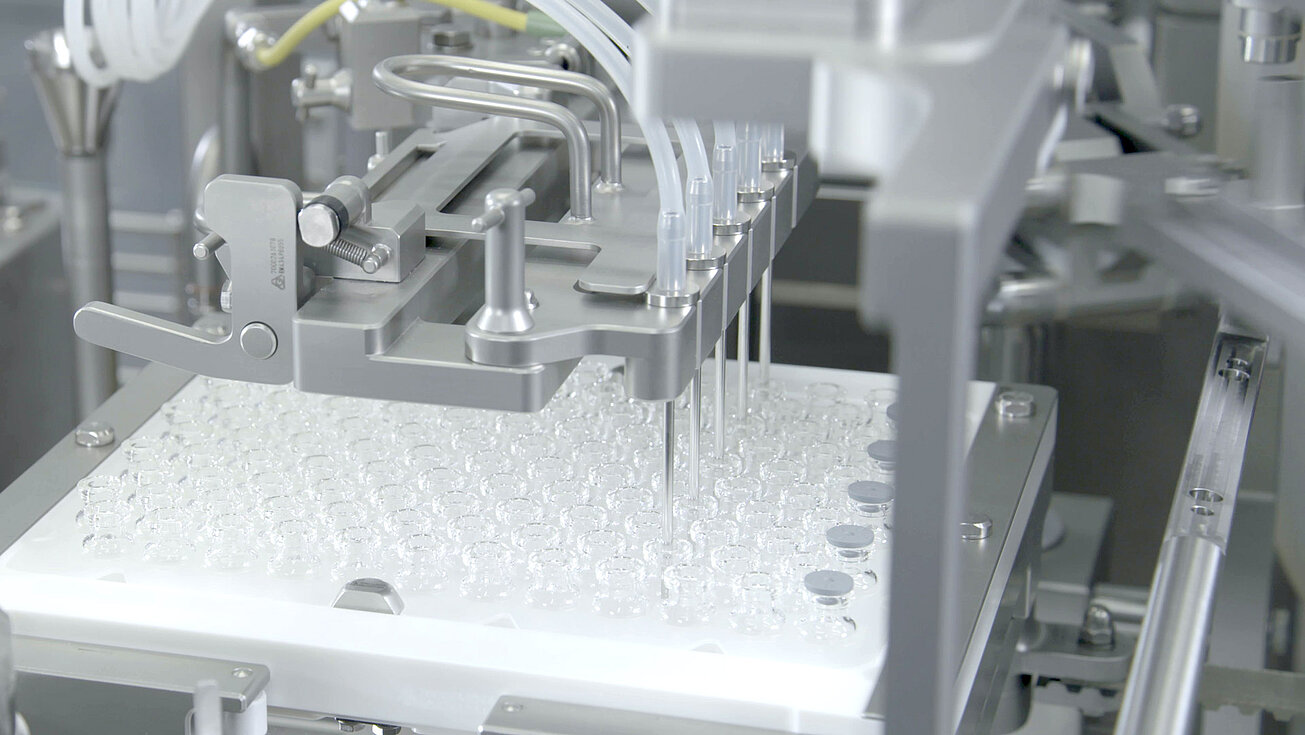
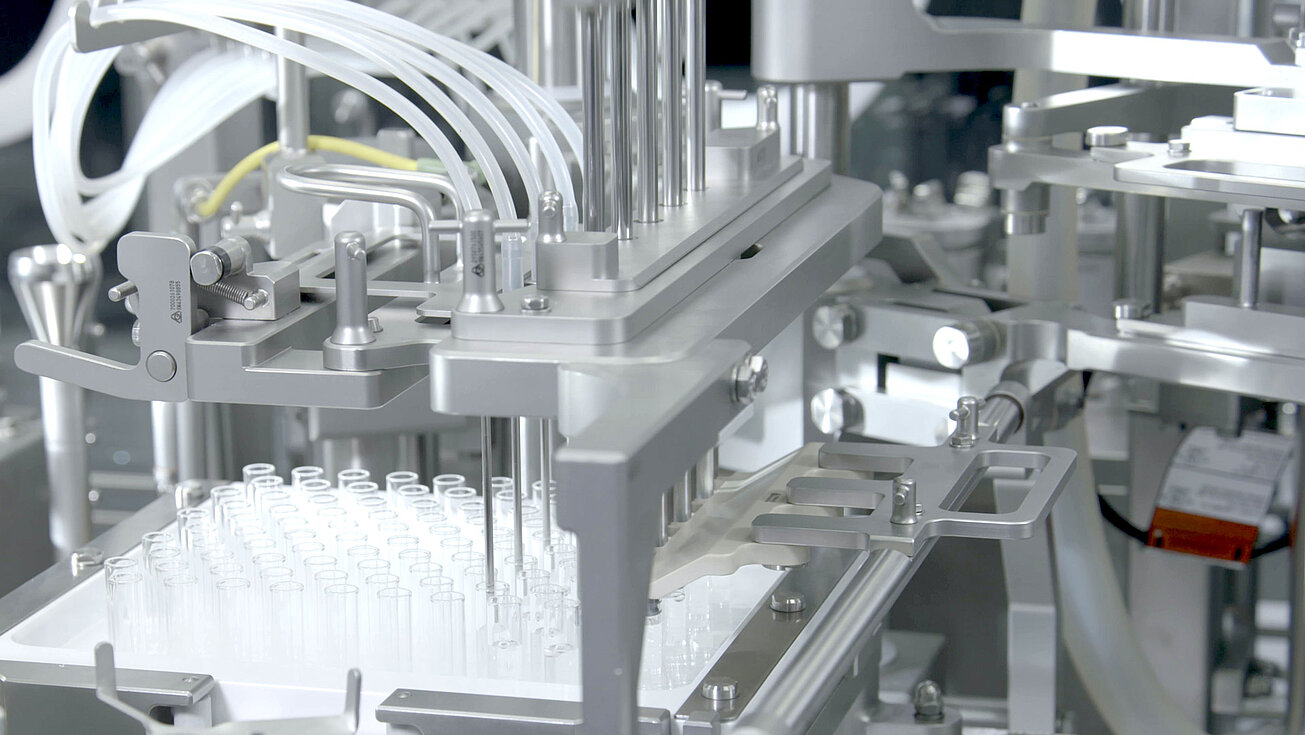
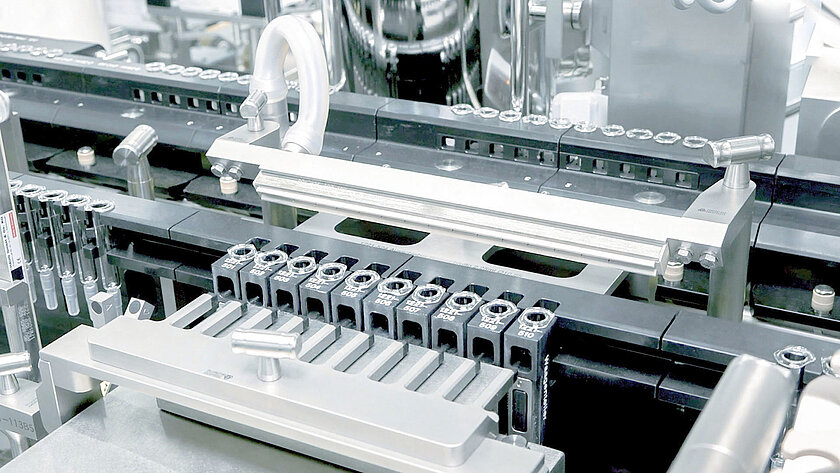
smarttrack
Universal sorting bowls
With our smarttrack technology, we ensure that the sorting of stoppers no longer takes place in the sorting bowls, but rather on the sorting tracks This means that a universal sorting bowl can be used for all types of stoppers instead of format-specific sorting bowls. Handmade sorting bowls with the associated long delivery times are a thing of the past.
innostream
Optimizing airflow
With innostream, groninger is breaking new ground in the field of GMP-compliant pharmaceutical machine construction. We made the optimal air flow priority. Innovative air flow on parts of the entire sorting unit that indirectly come into contact with the product is the core of our process solution. This reduces or even eliminates critical air pockets, uncontrolled air movements and the resulting undefined particle distribution.
fastlane
Increasing flexibility of the lines
Flexibility is one of the most important requirements for today's filling lines. With the fastlane approach, groninger tries to meet these requirements and designs format parts in such a way that delivery times can be significantly reduced.

Additional solutions for the efficient and safe processing of your pharmaceutical products
-
High degree of automation in a small space
Automation and good manufacturing practice also play an important role in research and development (R&D). groninger also offers automated solutions for small quantities, small spaces and laboratories.
-
Optimal machine operability
groninger machine concepts are usually designed for one-sided machine operation, consequently enabling 30% more effective operability with significant risk minimization.
-
End-to-end process solutions
groninger offers end-to-end process solutions that are scalable from R&D through clinical studies to various performance classes in the production environment.
-
Smallest number of format parts
Thanks to continuous functional and process optimization, the number of formats has been reduced by up to 40% compared to conventional filling lines.
-
Highest availability
The greatest possible automation of all production processes ensures that the system is available to the greatest extent - the OEE is maximized (downtime is minimized).
-
Scalable output
The scalable line concepts allow the output to be adjusted according to your requirements.