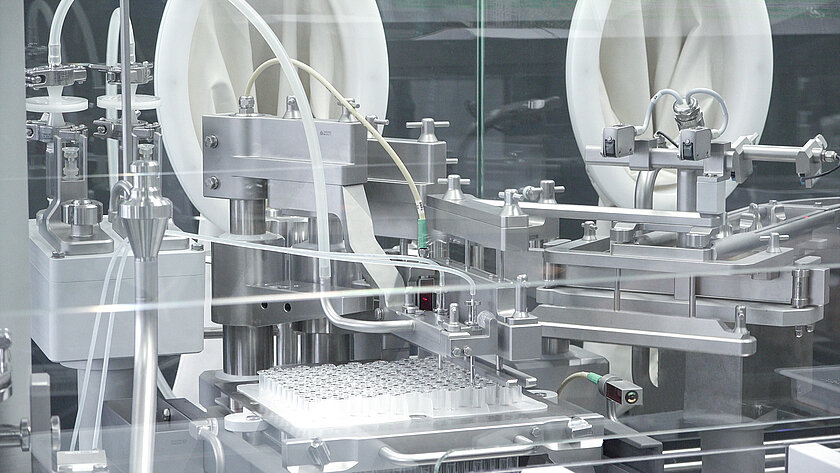
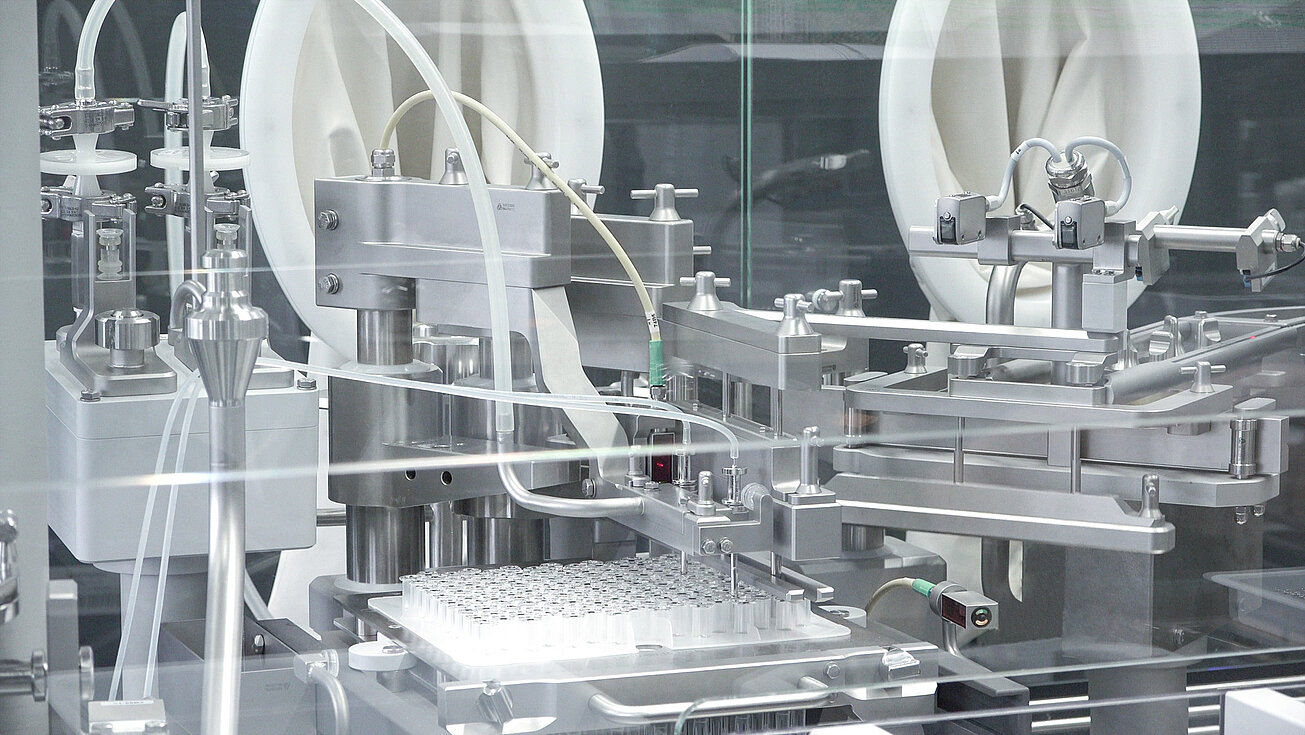
![[Translate to English:] Primärverpackungen [Translate to English:] groninger Primärverpackungen](/fileadmin/content/01_Pharma/04_Verarbeitungsobjekte/groninger-3in1_SW.jpg)
Processing pharmaceutical primary packages in nest and bulk
In the field of aseptic liquid filling, groninger machines mainly process syringes, vials and cartridges made of glass or plastic, but also eye drops and nasal spray bottles. Decades of experience and close cooperation with all well-known manufacturers of primary packaging make us a specialist in this field.
Ready-to-use packaging container
The ready-to-use syringe has been an essential packaging item in the pharmaceutical industry for over 30 years. It paved the way for additional RTU primary packaging such as vials and cartridges. With an estimated 5 billion RTU syringes on the market, this packaging represents by far the largest share.
The principle is simple, impressive and technically demanding: Pre-sterilized packaging is cleaned, assembled, placed in specially developed tub/nest configurations and sterilized. In these, they are protected from external influences through a liner and a lid in a single or double bag and can be transported sterile from production to the clean rooms without any problems.
For cartridges, in particular, there is the additional advantage that they can already be supplied with a crimp closure, which significantly simplifies subsequent processing.
Flexible use for all formats and batch sizes
By using RTU packaging with nest transport, today’s combination lines can do without complex format parts. This allows lines to be built and used with maximum flexibility and the shortest changeover times.
Thanks to COMBIFILL technology, groninger lines can already process RTU syringes, vials and cartridges as standard. The areas of application range from the processing of very small quantities for clinical studies or other small-batch filling to the filling vaccines on high-performance lines. groninger lines process between 5 and 1,000 packaging units per minute, depending on the configuration and requirements.
Non-sterile vials in bulk
Non-sterile vials are the primary packaging material most used in the pharmaceutical industry: Every year, around 20 billion vials are filled. With this type of packaging, non-sterile vials are delivered, which are then washed and sterilized on the line directly before filling.
This approach makes sense if only vials are processed in larger batches. Line changeovers are very complex with these primary packaging components. A change to syringes or cartridges is not possible.
A line is generally able to process between 60 and 400 units per minute.

Non-sterile syringes in bulk
In the past, so-called syringe bulk lines were used very frequently. For these lines, the syringes were delivered non-sterile and, similar to the processing of non-sterile vials, washed and sterilized directly on the line before they were filled and closed.
However, since syringes are not particularly stable, individual slides are required for them, which made processing very complex.
The use of pre-sterilized syringes combined with an increase in sales leads to significantly lower costs. Today, the processing of non-sterile syringes in bulk hardly plays a role.
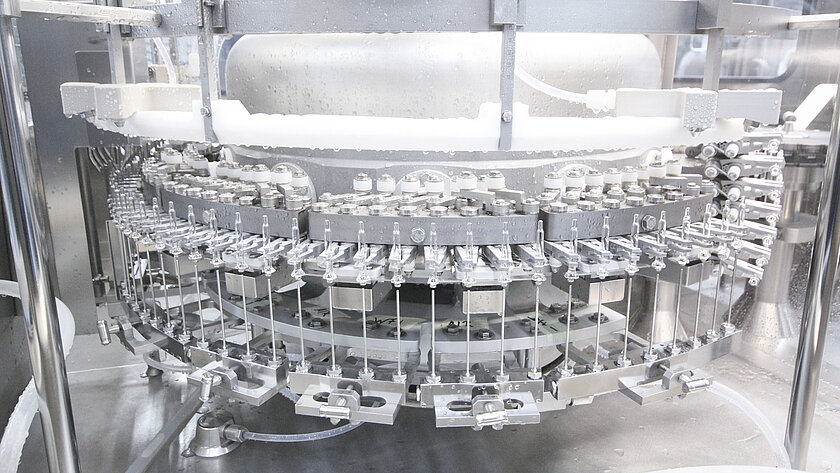
Eye drops and nasal spray bottles
Eye drops and nasal spray bottles are also increasingly processed as sterile bulk goods. The plastic packaging is delivered sterile and placed in the filling line via an aseptic transfer. When requirements for conventional eye and nasal drops need to be met, between 200 and 400 units can be filled and processed per minute. In addition to lines in oRABS, eye drops are also increasingly being used under isolator technology.


Packaging: Our suppliers and partners
Through close cooperation with various packaging material suppliers, we guarantee constant innovations in our filling and sealing systems for the benefit of our customers. We work with the following companies, among others:
- BD - Becton Dickinson
- Gerresheimer AG
- Nipro Group
- Schott Pharma
- Stevanato Group
- SiO2 Materials Science
- West Pharmaceutical Services