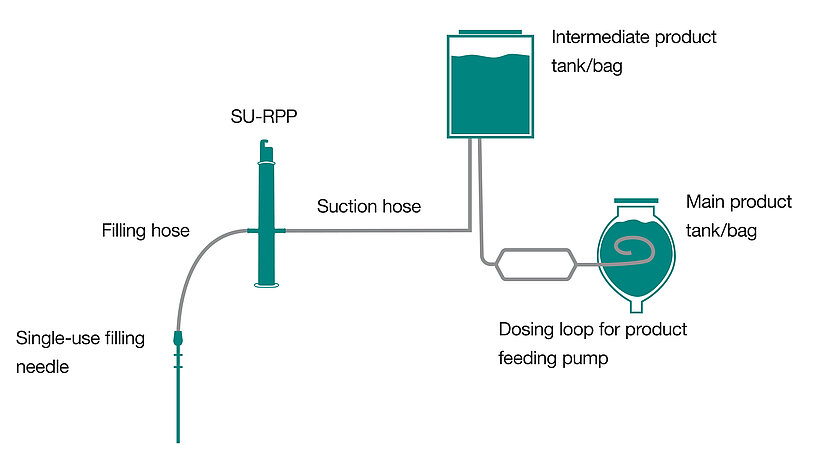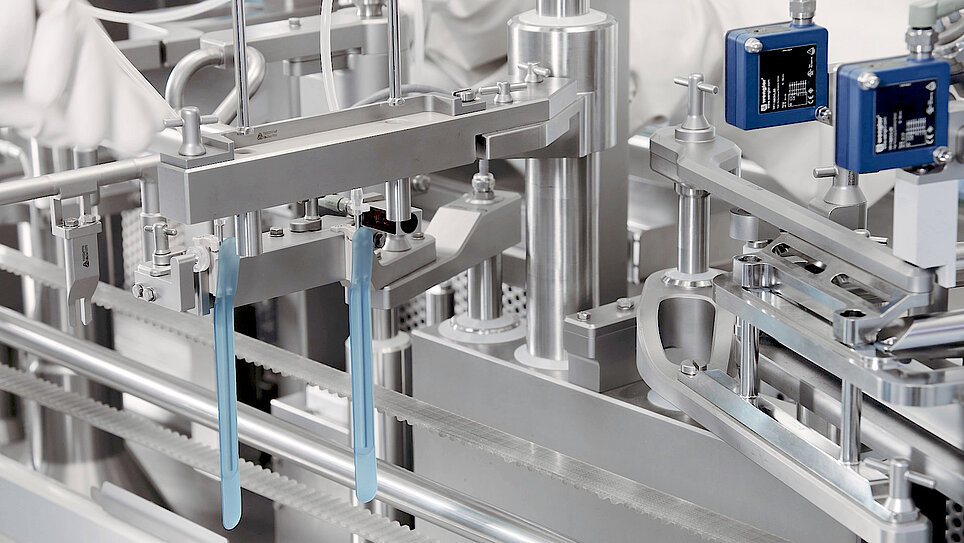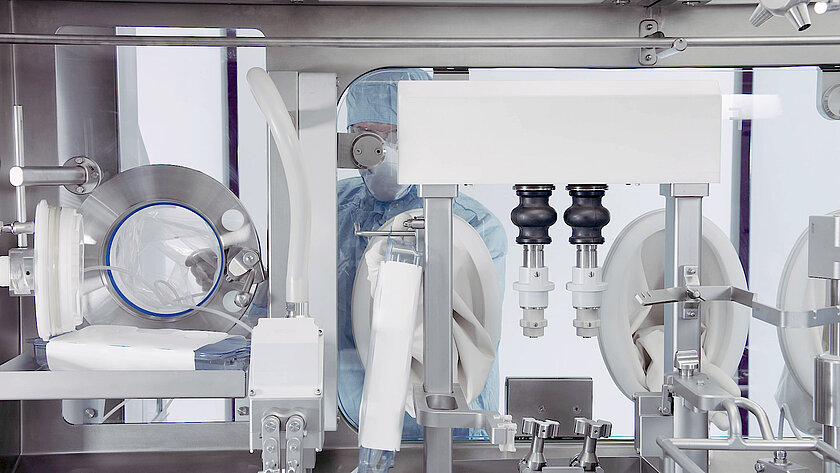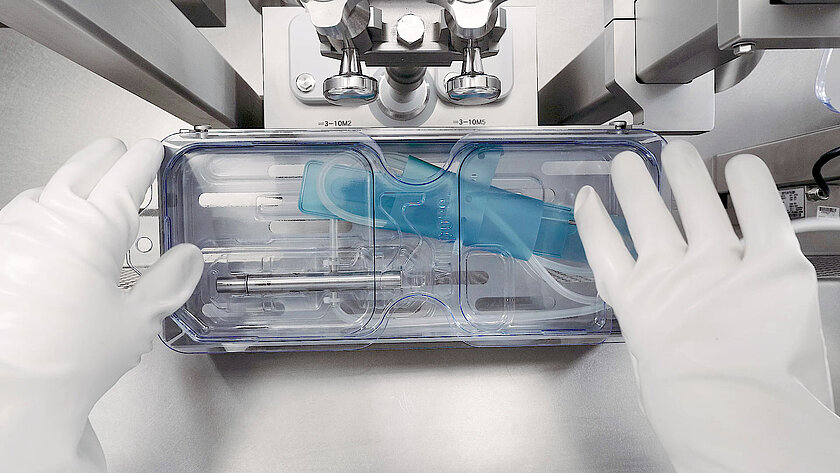
Single-Use Filling Needles
Increase production while reducing risk and utility consumption
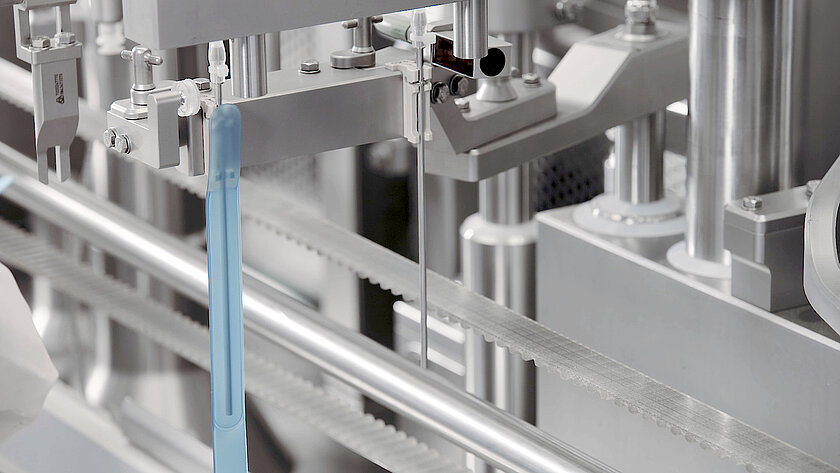
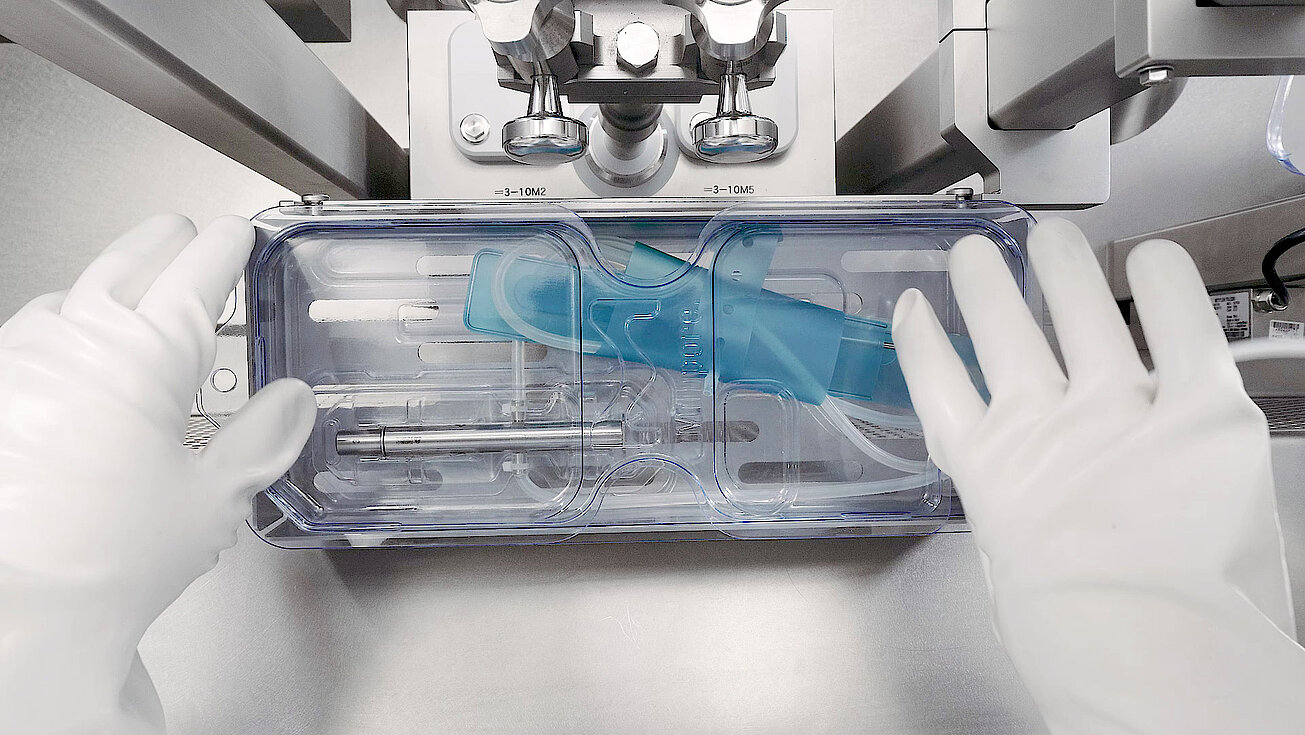
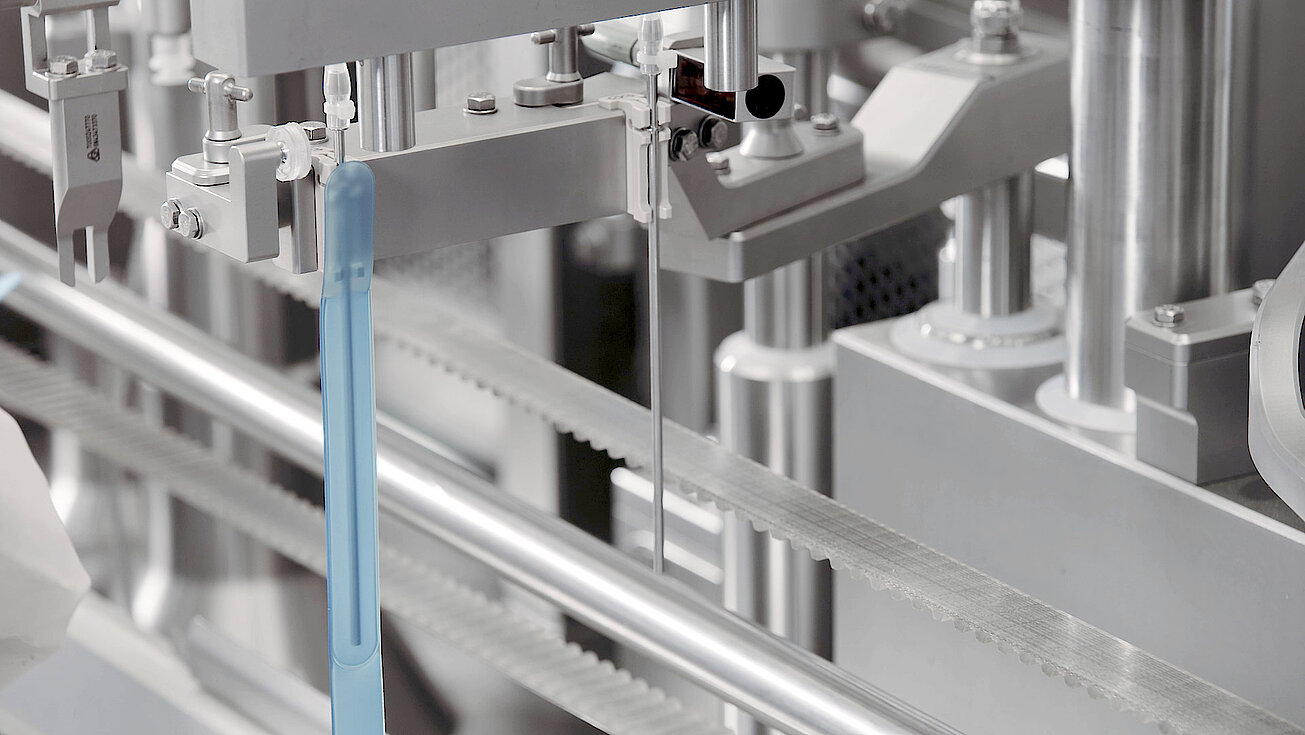
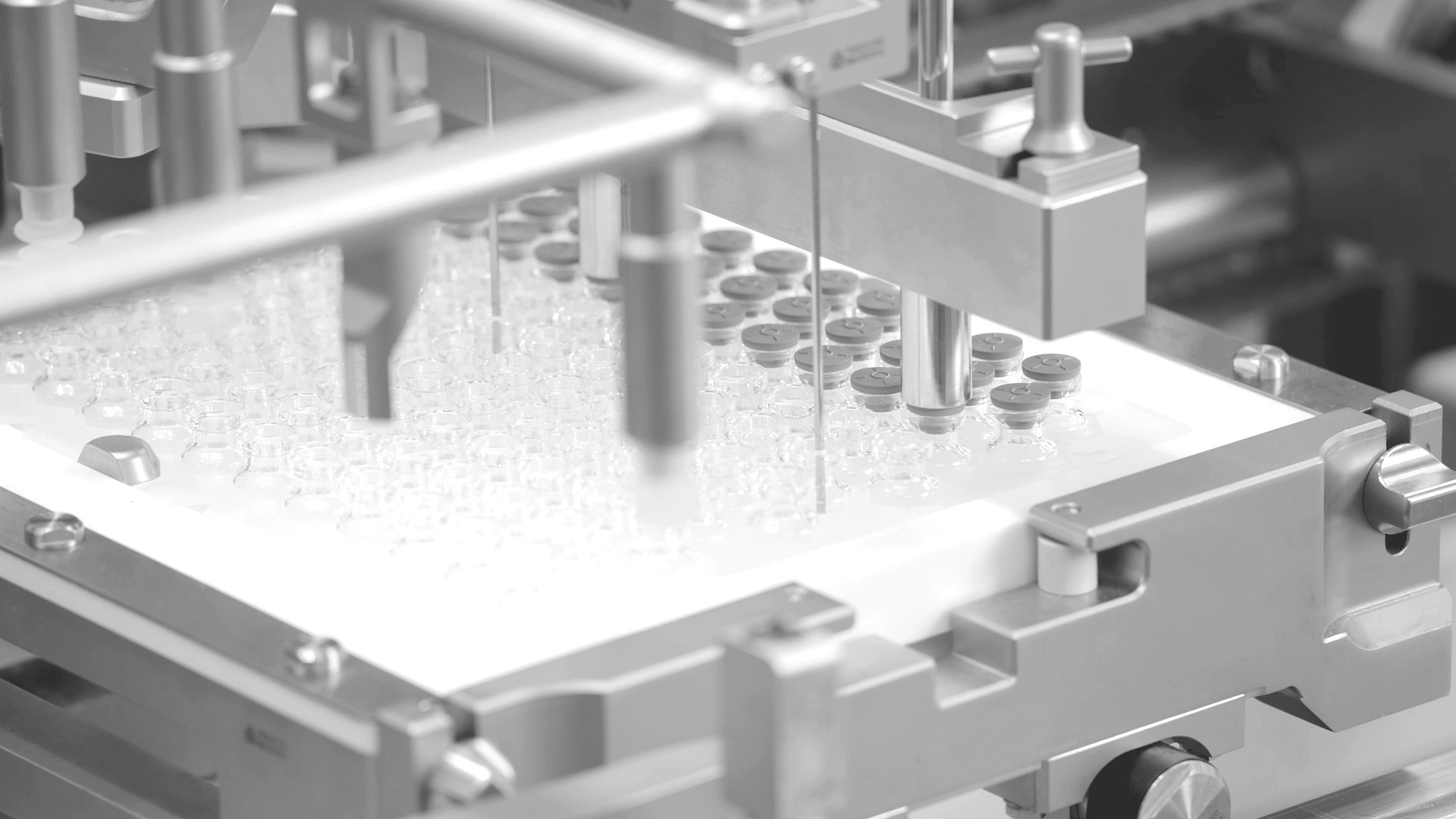
groninger single-use filling needles are available in different sizes, are compatible with groninger filling machines but can also be integrated by various machine vendors. Unlike multi-use needles, our single-use filling needles are provided cleaned and gamma irradiated, sterile and double packed. This eliminates the need for the end-user to perform any cleaning or sterilization of the needles. The needles are manufactured out of SST 316L/1.4435 according to DIN EN 10204 3.1. They have an inside and outside surface roughness of Ra 0.4 (except welding seams). With our best in-class single-use filling needle, we ensure optimum fill/finish results by establishing the highest quality standards.
Available in different sizes for a filling range from 0.1 ml – 100 ml
| Outer Diameter [OD] in mm | Inner Diameter [ID] in mm | Length in mm |
|---|---|---|
| 1.8 | 0.8 | 165 |
| 2.1 | 1.1 | 165 |
| 2.0 | 1.6 | 165 |
| 2.5 | 2.1 | 165 |
| 3.0 | 2.6 | 165 |
| 4.0 | 3.0 | 165 |
| 5.0 | 4.0 | 165 |
| 6.0 | 5.0 | 165 |
| 7.0 | 6.0 | 165 |
| 8.0 | 7.0 | 165 |
| 6.0 | 5.0 | 244 |
| 8.0 | 7.0 | 244 |
Benefits of Single-Use Filling Needles
- Eliminates the need for inline CIP/SIP
- Reduced CAPEX
- Reduced risk for cross contamination
- Closed system/process
- Increased machine availability
Our single-use filling needles can be integrated into a final fill set manufactured by a single-use equipment supplier, or directly ordered and integrated by the end-user.
The needles are validated which includes the following:
Cleaning
- Validation and revalidation of microbiological reduction (EN ISO 11737)
- LAL Endotoxin Reduction (USP 85, ANSI/AAMI ST 72)
- Particulate load (USP 788)
- Cytotoxicity (EN ISO 10993)
- Bioburden (EN ISO 11737)
Sterility
- Validation and revalidation of gamma sterilization (EN ISO 11137)
- Validation on sterile barrier (EN ISO 11607, ASTM F 1980)
- Transport validation (EN ISO 11607, ISTA 2A)
- Accelerated storage-testing (EN ISO 11607, ASTM F 1980)
Storage
- Temperature range: 10–25 ºC
- Humidity: 30–60%
- Sterilization method: Gamma (min. 25 kGy)
- Max. dose: 50 kGy
- Working temperature range: 5–45 ºC
- UV protected, avoid direct solar irradiation
Shelf life
- 5 years after packaging (sterility claim for barrier system)
Certificate
- Declaration of Conformity (DOC)